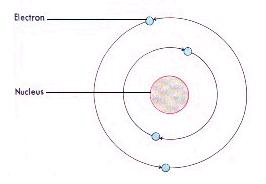
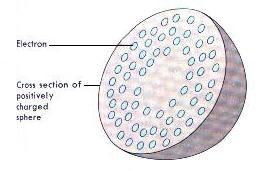
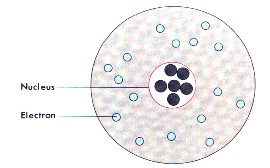
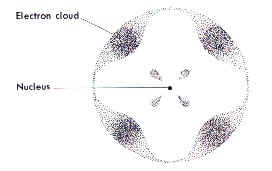
A Bunsen Burner is a device used in the laboratory for heating activities.
Conclusion: The tip of the inner blue flame is the hottest part of the Bunsen flame.
Parts
|
Functions
|
Air Holes
|
To allow air to
enter the burner
|
Barrel
|
To raise the
flame to a suitable height for heating/ burning
|
Base
|
To support the
burner and make it more stable
|
Collar
|
To control the
amount of air entering the burner
|
Gas Intake tube
|
To allow the
gas from the gas supply to rush into the burner
|
Gas Tap
|
To control the
amount of gas supplied to the burner
|
Aim: Find out which part of the *non-luminous flame is the hottest.
Procedure of experiment:
- Light the bunsen burner with a lighter adjusting the air holes to get non-luminous flame.
- Us the metal tongs to grab hold of the thin wires.
- Timing with the stopwatch, put the metal thin wires using the metal tongs form 1 minute at the tip of the inner blue flame.
- Repeat the step 2 and 3 for the inner blue flame and the tip of the outer blue flame.
- Compare the three wires to observe which wire has melted the most and the colour change of the wires.
Observation
Part of the flame
|
Observation
|
Inner blue
|
The copper wire
turned slightly red and melted slightly.
|
Tip of inner
blue
|
The copper wire
glowed brightly in red and it was melted almost halfway through
|
Tip of outer
blue
|
The copper wire
turned quite red and ¼ of it was
melted
|
Conclusion: The tip of the inner blue flame is the hottest part of the Bunsen flame.
 Bunsen burner |
 Non-luminous flame |
Strike back